Methane does not undergo this type of reaction because it is bounded with four hydrogen atoms while in ethane double bonds break and provide a site for addition. Whereas air contains less percentage.
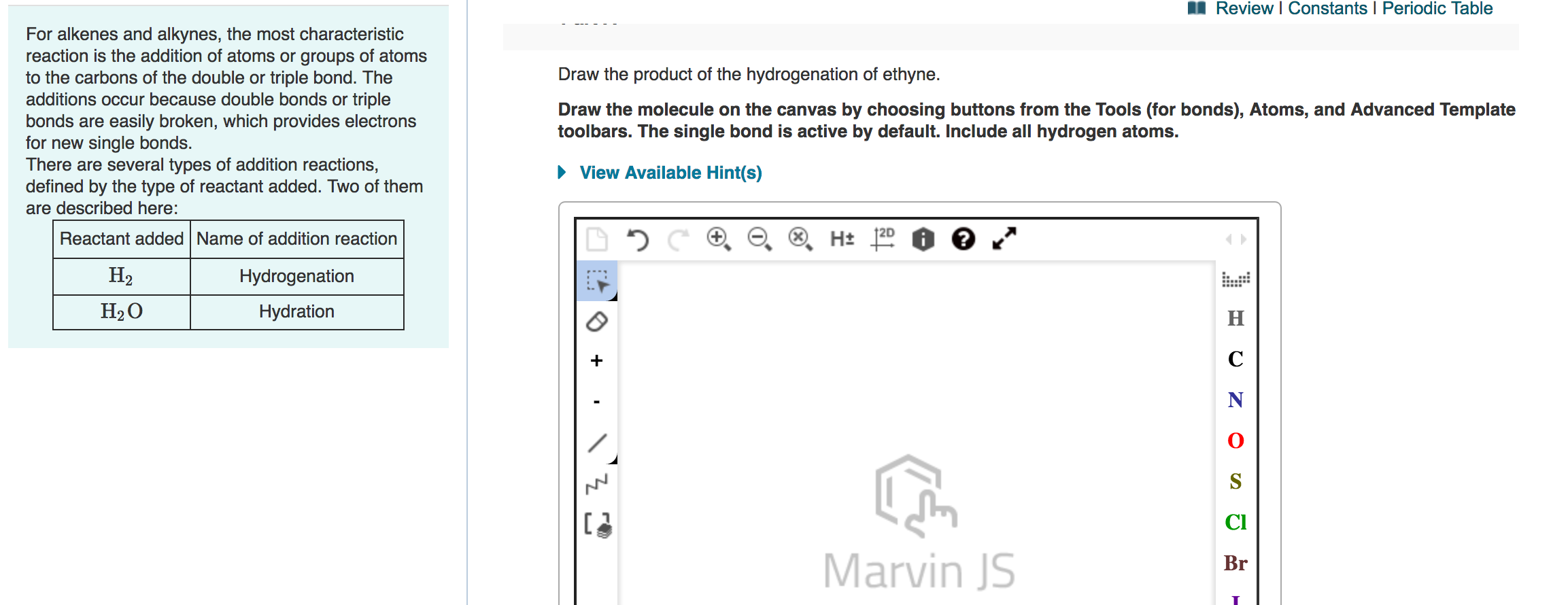
Solved A Review Constants Periodic Table Draw Chegg Com
Draw fully displayed structural formulae of the first four members of the alkenes and the products of their addition reactions with hydrogen water chlorine bromine and iodine.
. Alkenes may be turned into alkanes by reacting the alkene with hydrogen gas at a high temperature and high pressure. Email protected thermophysicalProperties New const dictionary dict Return a pointer to a new thermophysicalProperties. Carboxylic acids and esters are not easily reduced by catalytic hydrogenation or by NaBH 4.
Thus in ethyne molecule hydrogen. IN order for a reaction to produce a racemic mixture the product formed must possess at least one chiralOrganic chemistry is a branch of chemistry that studies the. Due to the maximum percentage of s character 50 the sp hybridized orbitals of carbon atoms in ethyne molecules have highest etcetronegativity.
Q27 What is the empirical formula of a compound 02801 gm of which gave on complete combustion Q30 0275 gm of an organic compound gave on complete combustion 022 gm of carbon dioxide and 0135. Ethyne In the above structure both carbons are bonded with triple bonds. Draw the structural formula for each of the.
Which attracts the shared pair of the C-H bond of ethyne to a greater extent than that of the sp 2 hybridized orbitals of carbon in ethene and the sp3 hybridized orbital of carbon in ethane. When methane is burnt in excess of air or oxygen with pale blue flame it gives carbon dioxide gas water and heat energy. Which attracts the shared pair of the C-H bond of ethyne to a greater extent than that of the sp 2 hybridized orbitals of carbon in ethene and the sp3 hybridized orbital of carbon in ethane.
Due to the maximum percentage of s character 50 the sp hybridized orbitals of carbon atoms in ethyne molecules have highest etcetronegativity. In your opinion why cannot we use a mixture of ethyne and air for this purpose. 57 Predict the product of the For the following SN1 reaction draw the major organic product identify the nucleophile substrate and leaving group and determine the rate limiting step.
Snyder Jon Antilla. Explain laboratory test of alcohols and phenols. A o-nitrophenol b o-hydroxy benzoic acid.
The general formula for the homologous series of alkenes is CₙH₂ₙ. Thus in ethyne molecule hydrogen. Academiaedu is a platform for academics to share research papers.
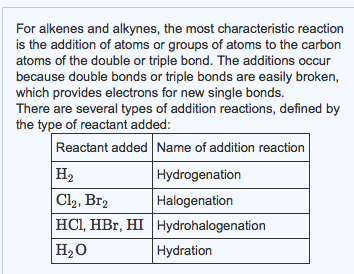
Addition reactions are common to both these compounds. Graham Solomons Craig B. A nickel catalyst is also needed to accomplish this addition reaction.
Eg ethene reacts with hydrogen to give ethane This reaction is also called saturation of the double bond. Draw the electron-dot structure for ethyne. Draw intramolecular hydrogen bonding structures in the following compounds.
Fryhle Scott A. Decane C10H22 CID 15600 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazards Property Name Property Value Reference. To understand the chapter hydrocarbons you have to study the chapter.
Study Guide and Solutions Manual to Accompany TW. In pure oxygen ethyne undergoes complete combustion and high temperature suitable for welding is attained. B o-hydroxy benzoic acid.
4721 Structure and formulae of alkenes. The complete and accurate NCERT Solutions for Class 11 Chemistry Chapter 13 will be updated soon Download NCERT Solutions Class 11 Chemistry Chapter 13 PDF Organic chemistry is the scientific study of the structure properties composition reactions and synthesis of organic compounds. A mixture of ethyne and oxygen is burnt for welding.
This reaction is complete oxidiation reaction. E Conversion of Methane to Ethyne C 2 H 2 When methane is heated to about 1500C in an electric arc and then suddenly cooled the product is C 2 H 2 and Hydrogen. Calculate the standard enthalpy of formation of c2h4 from the following thermochemical equation.
In ethene the carbon atoms are said to be unsaturated. Alkenes are hydrocarbons with a double carbon-carbon bond.
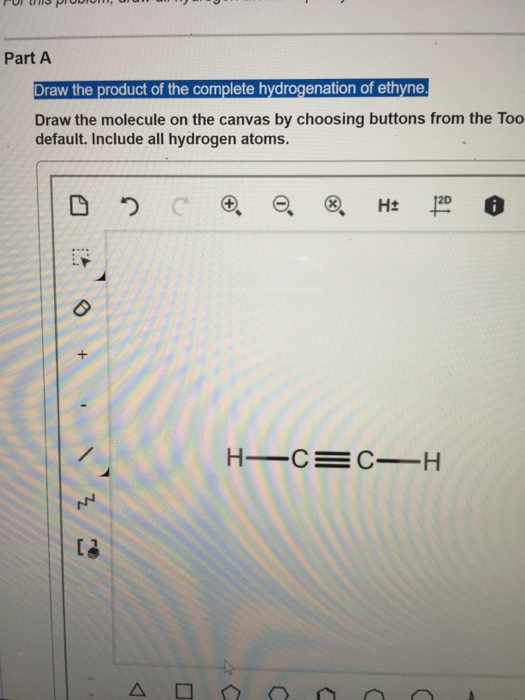
Solved 1 Draw The Product Of The Complete Hydrogenation Of Chegg Com

Answered Draw The Product Of The Hydrogenation Bartleby

Solved Methyl Shit Ch 20 Carbocation Draw The Reactant Chegg Com

Solved Draw The Product Of The Complete Hydrogenation Of Chegg Com

Solved 10 Of 13 Review I Constants 1 Periodic Table Part A Chegg Com
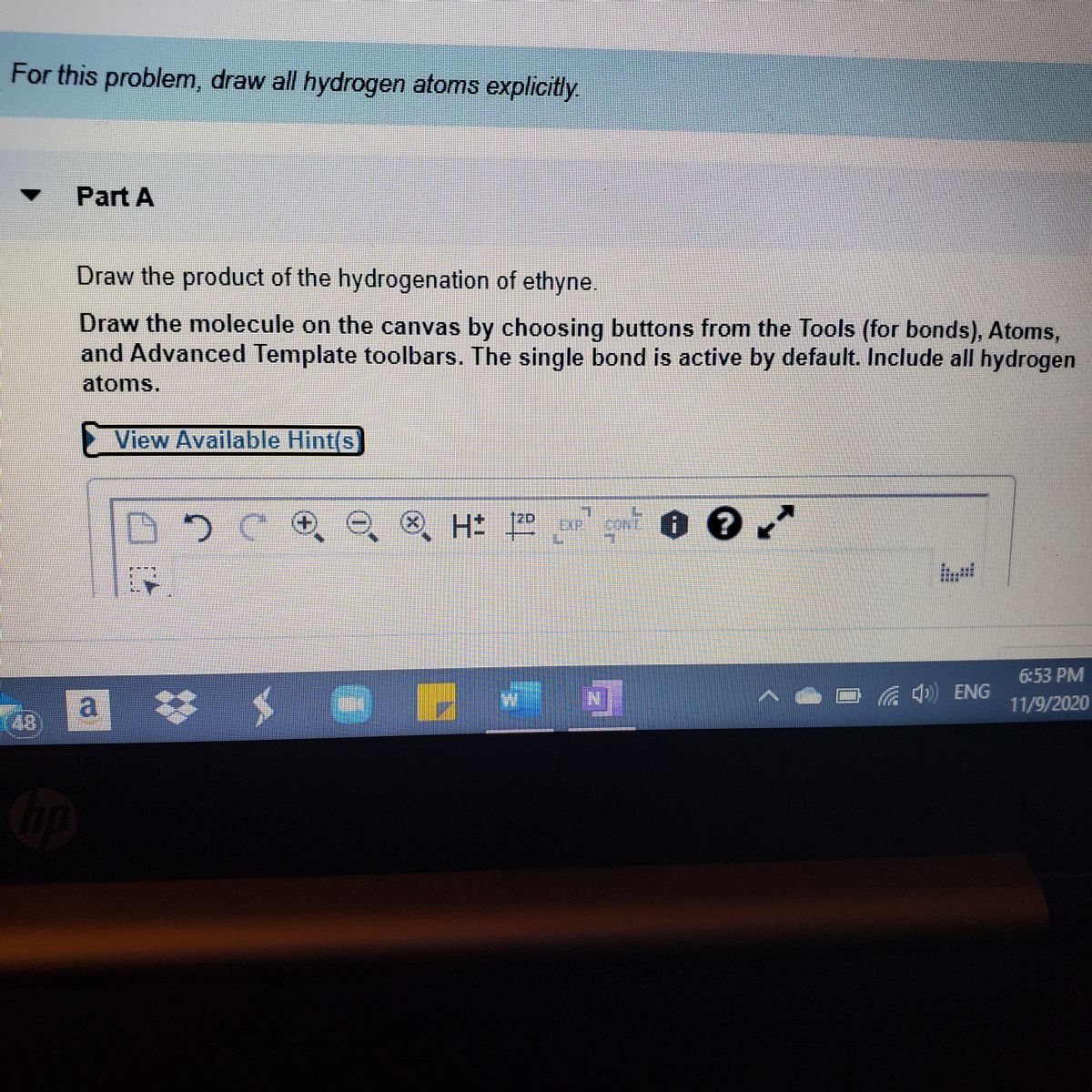
Answered Draw The Product Of The Hydrogenation Bartleby

Complete Hydrogenation Of Ethyne To Ethane Conversion Of Ethyne To Ethane Youtube
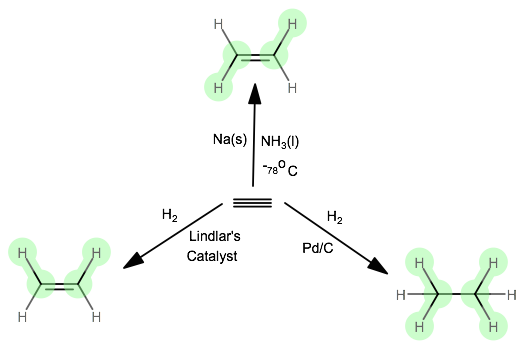
How Would You Draw The Product Of The Hydrogenation Of Ethyne Socratic
0 comments
Post a Comment